There are several types of “no touch” disinfection (NTD) systems that are commercially available for use in healthcare today. These systems can be divided into two major categories, aerosolized-vapor systems, and ultraviolet C radiation systems.
Aerosolized-Vapor Generating Systems
These systems are based on generating and spreading small disinfectant-containing particles throughout patient care environments (patient rooms, operating rooms, etc.). These particles then land on environmental surfaces, establish contact with microorganisms on these surfaces and ultimately deactivate them. The two most common systems in this category are aerosolized hydrogen peroxide systems and vaporized hydrogen peroxide systems.
1. Aerosolized hydrogen peroxide systems (aHP)
When activated, these systems automatically produce pressure-generated aerosol that contains 5-6% hydrogen peroxide and <50 ppm silver. The disinfectant is spread into the environment through a machine that creates droplets which land on environmental surfaces and consequently deactivate organism on these surfaces.
Depending on the product, these droplets range in size from 0.5 to 10 μm. Disinfectant particles are spontaneously converted into harmless water and oxygen molecules upon completion of the disinfection process without any need for a neutralization step.
Studies using aHP systems demonstrated efficacy in reducing C. difficile and MRSA on environmental surfaces at healthcare facilities, but did not show complete eradication in clinical settings.
aHP is straightforward to use and less expensive than other NTD systems. Before the system is activated, vents and doors must be sealed. In addition, safety monitors must be used to monitor the concentration of hydrogen peroxide in the environment. Cycle time is typically 2 hours for a single cycle, but multiple cycles may be required. The aerosolization process does not allow for the homogenous (even) distribution of the disinfectant on surfaces.
Examples of these systems are ASP GLOSAIR, STERIS BioGienie, and Oxypharm Nocospray.
2. Vaporized hydrogen peroxide systems
These systems deliver heat-generated hydrogen peroxide vapor through high-velocity air steam to achieve homogenous particle distribution throughout an enclosed area. These systems use hydrogen peroxide at levels which are EPA-registered as sterilants, sporicidal, bactericidal, mycobactericidal, and fungicidal.
Studies demonstrated that these systems effectively remove C. difficile, MRSA and S. aureus, MDR Gram-negative organisms and other pathogens from environmental surfaces.
HPV is less straightforward than aHP and UVC because it requires two units (a generator and an aeration unit). Doors and air vents must be sealed during the process. Safety monitors are required to ensure that no disinfectant leakage occurs during the process and to verify that the concentration of HP inside the room is below health and safety exposure limits. The cycle times are typically 1.5 hours for the Bioquell (HPV) and 8 hours for the Steris systems (VHP).
Ultraviolet C radiation (UVC)
Mercury-based UVC
These UVC systems deliver specific doses of UVC to environmental surfaces typically in the range of 254 nm. The dose required to kill spores is approximately 2-3 times larger than that used to deactivate vegetative cells (22,000-36,000 vs.12,000 uWs/cm2). Radiation moves in straight lines which makes it ineffective on surfaces that are outside the radiation range. This makes it necessary to move the system around the space to decontaminate several times to ensure that all high-touch surfaces are decontaminated.
These systems showed a 2-4 log10 reduction of healthcare pathogens but the elimination of key healthcare pathogens was incomplete. It was suggested that increasing the cycle time may increase the effectiveness of these systems.
The key advantage of using UVC systems is ease of use. They do not require door and vent sealing, and also require a relatively short cycle time. However, studies demonstrated incomplete deactivation of pathogens on environmental surfaces when using UVC. The inconsistency in delivering an adequate dose of radiation to all surfaces makes it necessary to manually move the device to different locations within the space to achieve full coverage of high-touch surfaces. In addition, it is necessary to administer more than one radiation dose to achieve adequate pathogen removal. This makes these systems operator dependent which may introduce an element of error in the process.
Examples: Lumalier, Tru-D
Pulsed-xenon ultraviolet (PX-UV)
These systems emit broad spectrum UV in short pulses (camera flashes). The machine cycle must be run in multiple room locations to ensure that all high-touch surfaces are covered; however the machine has a relatively short cycle time (5-10 minutes).
One study showed that the PX-UV system achieved a significant reduction in VRE contamination in a room in a 12 minute cycle.
These systems are very similar to the mercury light based UVC systems as far as the need for moving the system to several locations within the room to achieve better coverage of high-touch surfaces. This is necessary to address the line-of-sight issues.
Example: Xenex
Other systems
- Gaseous ozone can achieve a high level of microbial inactivation. However, the requirement for high humidity is a practical limitation. Furthermore, ozone is toxic to humans, which makes it impractical to implement in healthcare.
- Chlorine dioxide is highly effective against a wide range of pathogens. However, safety and compatibility with materials makes it a poor choice for healthcare.
- Other fogging systems using a variety of disinfectants have limited use in healthcare at the present time.
Recent Study Results
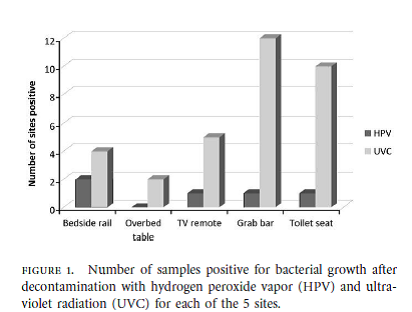
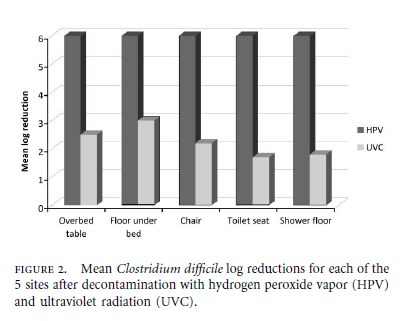
Below are highlights of a recent clinical study that compared the efficacy of a UVC to an HPV system.